Drug Eluting Stents

Drug Eluting Stents
CDSCO Medical
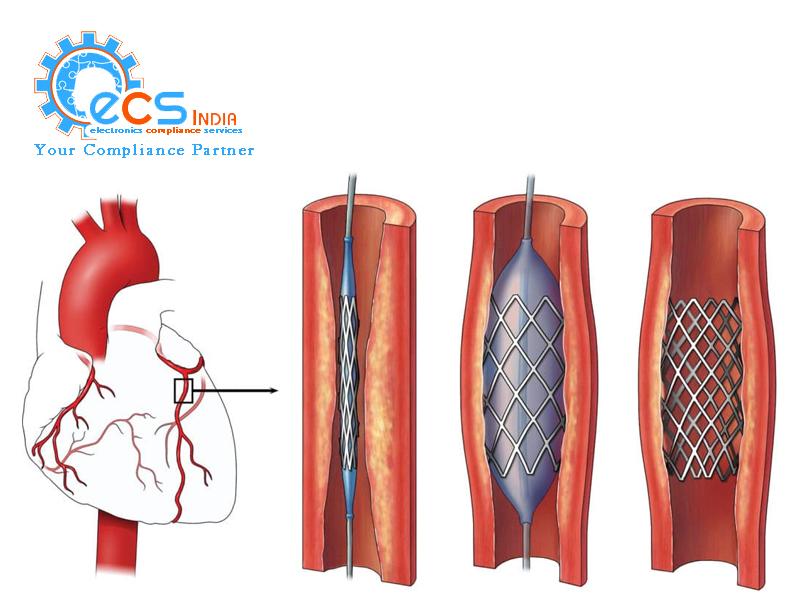
Drug-eluting stents (DES) are small mesh tubes made of metal that are used to treat blocked arteries in the heart. These stents are coated with medication that is slowly released into the bloodstream to prevent the formation of scar tissue and re-blockage of the artery. The Central Drugs Standard Control Organization (CDSCO) is the regulatory body responsible for regulating the manufacturing, testing, and approval of DES in India to ensure their safety, efficacy, and quality.
CDSCO Medical has established guidelines for the manufacturing, testing, and approval of DES in India. All manufacturers of DES must adhere to these guidelines and obtain a valid manufacturing license from CDSCO before producing and selling their products in India.
CDSCO requires all DES to undergo rigorous testing for their physicochemical properties, biocompatibility, and drug release characteristics before they can be approved for marketing and distribution. The testing must be conducted in accredited laboratories, and the results must meet the specifications outlined by CDSCO.
In addition, CDSCO has established guidelines for the labeling and packaging of DES to ensure their safe use by healthcare professionals. The labeling must include the name and address of the manufacturer, the composition of the stent, the drug used for coating the stent, and the precautions to be taken while using the stent.
CDSCO also plays a critical role in post-marketing surveillance of DES. The organization monitors adverse events associated with the use of DES and takes appropriate action, such as product recalls or warnings, to ensure the safety of patients.
It is important for healthcare professionals to follow the instructions provided by the manufacturer and CDSCO while using DES to ensure their safe and effective use. Patients should also be aware of the risks and benefits associated with the use of DES and should consult their healthcare providers before undergoing any procedures involving DES.
Overall, CDSCO plays a vital role in ensuring the safety, efficacy, and quality of DES used in medical procedures in India. Manufacturers, healthcare professionals, and patients must adhere to the guidelines established by CDSCO for the safe and effective use of DES.

3 Years Ragular
Ask Price
| Price: | As Per Client |
|---|---|
| Unit: | Services |
| Type: | Services |
Suggested Similar Drug Eluting Stents
Ask Price
We are one of the foremost manufacturers of premium
Ask Price
We are one of the foremost manufacturers of premium
Ask Price
We are one of the foremost manufacturers of premium
Ask Price
We are one of the foremost manufacturers of premium
Ask Price
We are one of the foremost manufacturers of premium
Ask Price
We are one of the foremost manufacturers of premium
Ask Price
We are one of the foremost manufacturers of premium
Ask Price
We are one of the foremost manufacturers of premium
Ask Price
We are one of the foremost manufacturers of premium
Ask Price
We are one of the foremost manufacturers of premium
Ask Price
We are one of the foremost manufacturers of premium
Ask Price
We are one of the foremost manufacturers of premium
Ask Price
We are one of the foremost manufacturers of premium
Ask Price
We are one of the foremost manufacturers of premium